
α-halo ketones when treated with a strong base (generally alkoxides) lead to the formation of esters with rearrangement of the carbon frame work.
- A base induced carbanion rearrangement
- Alkali hydroxides/amines give acids/amides.
- Cyclic ketones may result in ring contraction.
- The halogen is either chlorine or bromine, the presence of Iodine some times even bromine leads to SN2 reaction.
- Hydroxides as bases result in salts of carboxylic acids.
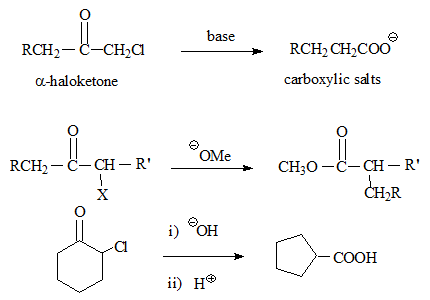
The mechanism
Through product studies it was proposed (by Favorskii himself) that the rearrangement resembles Benzyl -benzilic acid rearrangement (this mechanism had been established at that time)
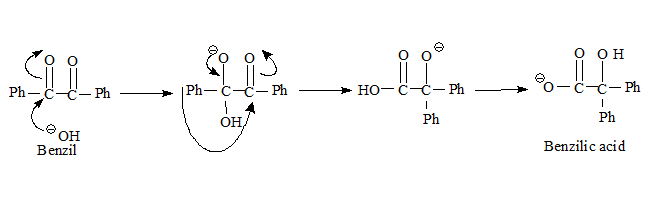
- The base attacks the carbonyl carbon resulting in an anion which through phenyl migration forms the anion of benzylic acid.
- Subsequent acidification can result in the acid itself
Applying this to Favorskii rearrangement, Compound 1 and 2 should give the corresponding products as shown below.
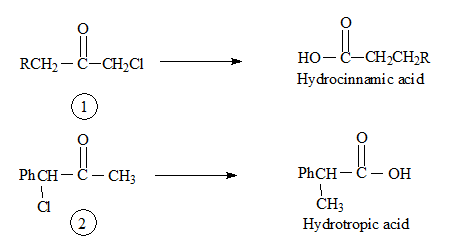
In actual fact both compounds give the same product hydrocinnamic acid. Hence it was thought that some other mechanistic pathway to be operative.
Loftfield Mechanism
The University of New Mexico Department of Biochemistry & Molecular Biology
Loftfield, in 1951 described a pathway that goes through a cyclopropanone derivative as an intermediate which is the most satisfactory general mechanism to date.
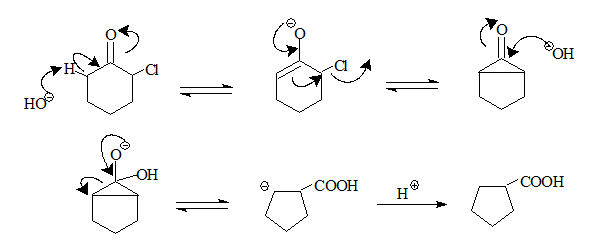
- The base abstracts a proton from the α carbon forming a resonace stabilised enolate anion.
- The anion through a internal SN2 displacement of the halogen forms a cyclopropanone intermediate.
- The intermediate is then attacked by the base at the carbonyl carbon
- Cyclopropanone ring opening and protonation results in the carboxylic acid.
- Aloxides as bases result in esters.
Applying this mechanistic route to the reactions involving compound 1 and 2.
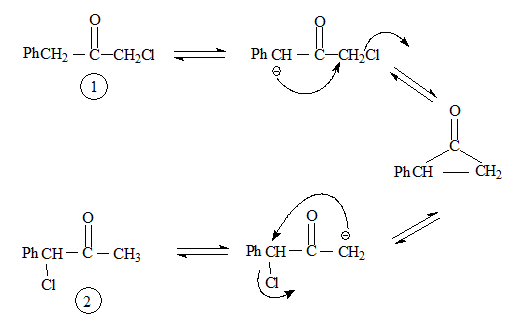
Both go through the same intermediate hence the same product.
It is possible for an anion to be formed at the second α-carbon, but the presence of halogen would destabilise it.
So far the mechanistic probe of product identification has been used. This was then confirmed by extensive isotopic labelling studies. Some of those are described in the following schemes.
Synthesis labelled α-chloro cyclohexanone.
Degradation studies. Identification and estimation of the labelled carbon in the products.